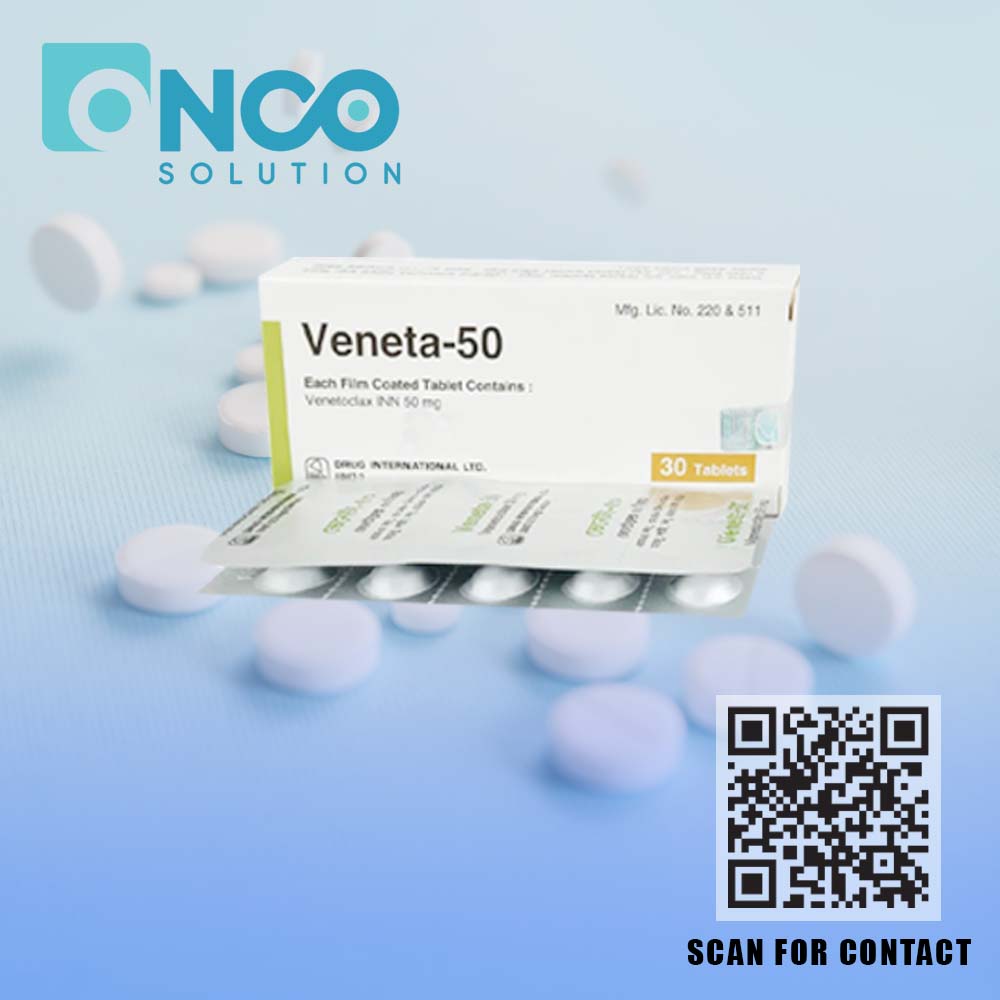
Veneta 50 mg (Venetoclax) – Precision Therapy for Chronic Lymphocytic Leukemia (CLL)
Veneta 50 mg (Venetoclax) is an advanced targeted therapy designed to treat chronic lymphocytic leukemia (CLL). As a BCL-2 inhibitor, Venetoclax works by restoring programmed cell death (apoptosis) in leukemia cells, effectively reducing their survival and stopping disease progression. This innovative approach has significantly improved treatment outcomes, offering patients a better quality of life.
Manufactured by Drug International Ltd and supplied by Onco Solution, Veneta 50 mg is prescribed for adult patients with CLL, including those who have previously undergone treatment or have genetic risk factors such as 17p deletion or TP53 mutation. This medicine can be used alone or in combination with other therapies like Rituximab, depending on the patient’s condition.
How Veneta 50 mg Works
Venetoclax works by selectively inhibiting BCL-2, a protein that prevents cell death in cancerous B-cells. Overexpression of BCL-2 is a hallmark of CLL, allowing leukemia cells to thrive and resist standard treatments. By blocking BCL-2, Veneta 50 mg induces mitochondrial outer membrane permeabilization (MOMP), which activates caspases and restores normal cell death. This mechanism makes it highly effective in eliminating leukemia cells and preventing disease progression.
Clinical studies have demonstrated that Venetoclax significantly reduces tumor burden and helps patients achieve long-term remission. Its precision-targeted approach ensures that healthy cells are minimally affected, reducing the side effects commonly seen with traditional chemotherapy.
Indications for Use
Veneta 50 mg is indicated for the treatment of chronic lymphocytic leukemia (CLL) in the following cases:
- Combination Therapy – Used with Rituximab in patients who have received at least one prior therapy.
- Monotherapy for High-Risk Patients – Recommended for patients with 17p deletion or TP53 mutation who cannot tolerate or have failed a B-cell receptor pathway inhibitor.
- Monotherapy for Other CLL Patients – For patients without 17p deletion or TP53 mutation, Venetoclax is used if they have failed chemoimmunotherapy and a B-cell receptor pathway inhibitor.
Dosage & Administration
Veneta 50 mg should always be taken under medical supervision, following a structured dosing regimen:
- Starting Dose – 20 mg once daily for 7 days.
- Dose Titration – The dose gradually increases over 5 weeks to minimize the risk of tumor lysis syndrome (TLS). The final maintenance dose is 400 mg per day.
- Combination with Rituximab – Once titration is complete, 400 mg daily is administered alongside Rituximab for a total treatment duration of 24 months.
- Monotherapy – 400 mg daily until disease progression or intolerance.
To ensure the best absorption and minimize side effects, Veneta 50 mg should be taken with food and water. Patients should also follow strict hydration guidelines to reduce the risk of tumor lysis syndrome (TLS).
Side Effects of Veneta 50 mg
Venetoclax is well-tolerated, but some patients may experience side effects. Common side effects include:
- Neutropenia (low white blood cell count)
- Diarrhea, nausea, and vomiting
- Anemia (low red blood cell count)
- Fatigue and weakness
- Upper respiratory tract infections
In some cases, serious side effects such as pneumonia, febrile neutropenia, and tumor lysis syndrome (TLS) may occur. TLS is a life-threatening condition caused by the rapid breakdown of cancer cells, leading to electrolyte imbalances. Patients at high risk should be closely monitored during dose escalation.
Drug Interactions
Veneta 50 mg is metabolized by the CYP3A enzyme, and its effectiveness can be affected by drug interactions. Patients should be cautious about the following:
- CYP3A Inhibitors – Strong inhibitors like ketoconazole and ritonavir can increase Venetoclax exposure, leading to toxicity. These medications should be avoided.
- CYP3A Inducers – Strong inducers like rifampin can reduce Venetoclax effectiveness and should not be used.
- P-gp and BCRP Inhibitors – These can increase Venetoclax levels, requiring careful monitoring.
- Food Interactions – Patients should avoid grapefruit, Seville oranges, and starfruit, as they contain CYP3A inhibitors that can interfere with Venetoclax metabolism.
Contraindications & Warnings
Veneta 50 mg should not be used in the following cases:
- Hypersensitivity to Venetoclax or its excipients
- Concurrent use of strong CYP3A inhibitors during dose titration
- Concomitant use of St. John’s Wort, which can reduce Venetoclax effectiveness
Special precautions should be taken for:
- Tumor Lysis Syndrome (TLS) – Patients must be monitored closely during dose escalation. Preventive hydration and uric acid-lowering medications may be required.
- Neutropenia – Regular blood tests are necessary due to increased infection risks.
- Hepatic & Renal Impairment – Patients with severe impairment should be closely monitored, with potential dose adjustments.
- Immunization – Live vaccines should be avoided during and after treatment until immune function recovers.
Use in Special Populations
- Pregnancy & Lactation – Venetoclax is contraindicated during pregnancy due to potential fetal harm. Effective contraception is required during and for 30 days post-treatment.
- Pediatric Use – Safety and efficacy in children under 18 years have not been established.
- Elderly Patients – No dose adjustment is required for patients over 65 years.
- Renal & Hepatic Impairment – Close monitoring is necessary, with potential dose modifications.
Why Choose Onco Solution?
Onco Solution is a trusted global supplier of oncology medicines, ensuring reliable access to Veneta 50 mg (Venetoclax) for healthcare providers and patients worldwide. With a strong commitment to cancer care, Onco Solution delivers high-quality, genuine medications with professional service and support.
For more information or to place an order, contact Onco Solution today.